So much for the rows. Beryllium Be Magnesium Mg Calcium Ca Strontium Sr Barium Ba Radium Ra For detailed information on alkaline earth metals read the main article on Alkaline earth metals on periodic table.
The Periodic Table Reading The Periodic Table Sparknotes
First there is an integer whole number in some part of the box.

. The entir table is a box of many boxes. What is group A and B in periodic table. The elements from Group 3 to 12 are called Transition Metals.
Classified as an alkaline earth metal it is located in group or column 2 on the periodic table along with beryllium magnesium calcium strontium and radium. How to Identify It. The periods are often defined as recurring sets of behavior.
Groups are labeled at the top of each column. Transition Metals are hard and dense are good conductors of heat and electricity and can be bent easily. Each box represent a given element.
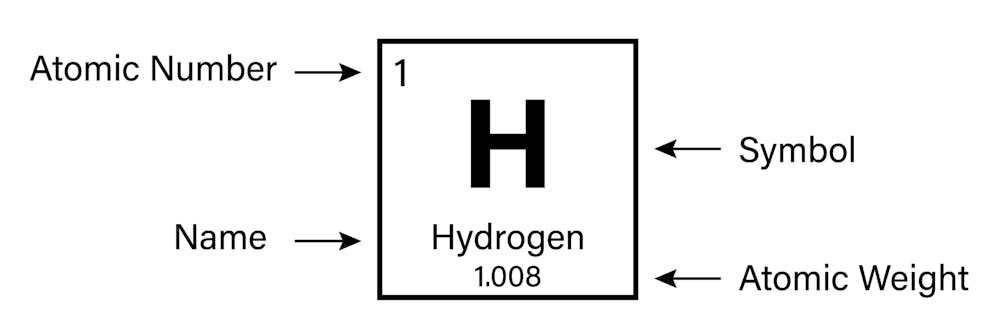
There is a sequencial number indicating the element by the number of protons in the nucleus and a second number hovering around twice as much indicating the number of protons and neutrons present the atomic mass indicating the isotope of the element. Each box represents an element and contains its atomic number symbol average atomic mass and sometimes name. Most periodic tables do not number them because they are fairly obvious but some tables do.
The one exception is the f. Where is the atomic. They include the Scandium Titanium Vanadium Chromium Manganese Iron Cobalt Nickel Copper and Zinc families of elements.
Alkaline earth metals are the group 2 elements on the periodic table. The period indicates the highest energy level attained by electrons of an atom of the element in the ground state. There are 118 elements known and hence there are 118 boxes in Each box represent a given element.
Properties of barium Pure barium is a soft silvery white metal. See how the atomic number increases by one for. The rows in the periodic table are called periods.
When the Russian chemist Dmitri Mendeleev came up with the modern periodic table there were several versions before this many elements werent even discovered. The elements included in Alkaline earth metals group are. The elements are arranged in seven horizontal rows called periods or series and 18 vertical columns called groups.
The elements are arranged in seven horizontal rows called periods or series and 18 vertical columns called groups. We have seven periods in the periodic table the two bottom rows are actually continuations of the 6th and 7th periods The period number is usually found to the left of the first element box for each row. It represents the number of protons found in the nucleus of one atom of that particular element.
There are 118 elements known and hence there are 118 boxes in the modern periodic table. How many boxes are there in the periodic table. Answer 1 of 5.
What about the columns. How many boxes are there in the periodic table. The rows of the periodic table are called periods.
119 rows In the periodic table the vertical columns are called groups and the horizontal rows. They are arranged in proton ascending order. In the Periodic Table theyre known as Groups.
What is represented by 1 on the periodic table. This is the atomic number of the element. What do the boxes represent on the periodic table.
Period numbers are located on the left-hand side of the table. Each of their atoms contains two valence outermost electrons. Each box represents an element and contains its atomic number symbol average atomic mass and sometimes name.

How To Read The Periodic Table Overview Components Expii
The Periodic Table From Its Classic Design To Use In Popular Culture
0 Comments